When sourcing proton pump inhibitor (PPI) APIs, Omeprazole and Esomeprazole often stand at the top of the list for pharmaceutical manufacturers. Both are widely used to treat acid-related disorders such as GERD, peptic ulcer disease, and erosive esophagitis. While these molecules appear similar, their scientific, regulatory, and commercial differences can significantly impact formulation performance and market positioning.
For API importers, understanding these distinctions is essential to make informed sourcing decisions, ensure compliance, and build a reliable supply chain. This guide explores the key differences between Omeprazole API and Esomeprazole API, focusing on quality, stability, regulatory expectations, and commercial considerations.
Understanding the Molecules: Omeprazole API vs. Esomeprazole API
Omeprazole API – A Racemic Mixture
Omeprazole is a racemic mixture containing two enantiomers (R-omeprazole and S-omeprazole). Although effective and widely used, its mixed composition results in certain pharmacokinetic variations among patients.
Esomeprazole API – The Pure S-Enantiomer
Esomeprazole, on the other hand, is the S-enantiomer of omeprazole, developed to offer improved bioavailability, enhanced acid control, and more predictable therapeutic outcomes.
This single-enantiomer structure contributes to:
- Higher systemic exposure
- Reduced inter-patient variability
- More consistent acid suppression
Purity and Quality Requirements – Omeprazole API vs. Esomeprazole API
Omeprazole API Purity
Omeprazole typically has slightly lower enantiomeric purity because it contains both enantiomers. Specification compliance includes parameters such as:
- Related substances
- Residual solvents
- Enantiomeric purity
- Polymorphic form
- Assay (on anhydrous basis)
Esomeprazole API Purity
Esomeprazole requires significantly higher chiral purity, often ≥ 99.8% of the S-enantiomer. Because of its optical specificity, manufacturers must implement advanced chiral separation technology or optimized synthesis routes.
For importers, this translates to:
- Higher manufacturing sophistication
- Stricter regulatory scrutiny
- More detailed analytical documentation
Pharmacokinetics and Clinical Performance – Omeprazole API vs. Esomeprazole API
From a clinical perspective, Esomeprazole delivers better acid suppression, especially in patients with severe GERD, due to:
- Higher bioavailability
- More consistent plasma concentrations
- Longer duration of action
Omeprazole is effective in general therapy but may exhibit variable patient response due to its racemic nature.
For finished-dosage manufacturers, this means:
- Esomeprazole helps build a premium product segment
- Omeprazole is ideal for cost-effective, high-volume formulations
Stability Profiles and Storage Conditions – Omeprazole API vs. Esomeprazole API
PPIs are inherently light- and moisture-sensitive, requiring careful control.
Omeprazole API Stability
Omeprazole shows moderate stability under standard conditions but still requires:
- Light-protective packaging
- Desiccants
- Controlled humidity
Esomeprazole API Stability
Esomeprazole can be slightly more sensitive due to its enantiomeric purity. Many manufacturers produce it as:
- Esomeprazole magnesium
- Esomeprazole sodium
- Esomeprazole strontium
These salt forms enhance stability and improve formulation performance.
For importers, examining ICH stability data and shelf-life studies from the supplier is essential.
Impurity Profile and Regulatory Compliance – Omeprazole API vs. Esomeprazole API
Impurity limits significantly differ between the two APIs.
Omeprazole API Impurities
Being older and widely manufactured, Omeprazole has a well-defined impurity profile, including:
- Process impurities
- Degradation impurities
- Enantiomeric impurities
Most manufacturers comply with USP, EP, BP, and IP standards.
Esomeprazole API Impurities
Esomeprazole requires stringent control on:
- Chiral impurities (R-omeprazole)
- Organic impurities
- Residual solvents
Regulators expect detailed chiral method validation, which should be part of the supplier’s DMF.
Importer tip: Always request the latest DMF (Type II) or CEP for streamlined registrations.
Particle Size Distribution (PSD) and Formulation Impact
Both APIs require controlled PSD for optimal dissolution.
- Omeprazole PSD affects capsule performance and granule coating.
- Esomeprazole PSD is critical for delayed-release formulations.
Manufacturers typically provide:
- Micronized grade
- Customized particle size
- Stability-optimized particle engineering
Importers should evaluate PSD consistency across batches to ensure finished product uniformity.
Cost, Availability & Market Demand – Omeprazole API vs. Esomeprazole API
Omeprazole API Cost & Supply
Omeprazole is widely available, cost-effective, and produced by many global manufacturers.
It is ideal for:
- Generic formulations
- Emerging markets
- Large-volume production
Esomeprazole API Cost & Supply
Esomeprazole is comparatively premium-priced, due to:
- Higher synthesis complexity
- IP considerations in certain markets
- Lower global manufacturing capacity
It is preferred by:
- Regulated market manufacturers
- Companies targeting premium therapy segments
Regulatory Documentation for Importers – Omeprazole API vs. Esomeprazole API
Before importing, ensure the supplier provides:
- US-DMF / EU-DMF or CEP
- GMP certificate
- Stability data (accelerated + long-term)
- Detailed impurity profile
- Residual solvent report
- Chiral purity data (for Esomeprazole)
- Particle size distribution reports
- CoA for each batch
For regulated markets, additional documents may include:
- Validation reports
- Process flow diagrams
- TSE/BSE certificates
Thykn ensures full regulatory transparency with comprehensive GMP, documentation, and quality systems.
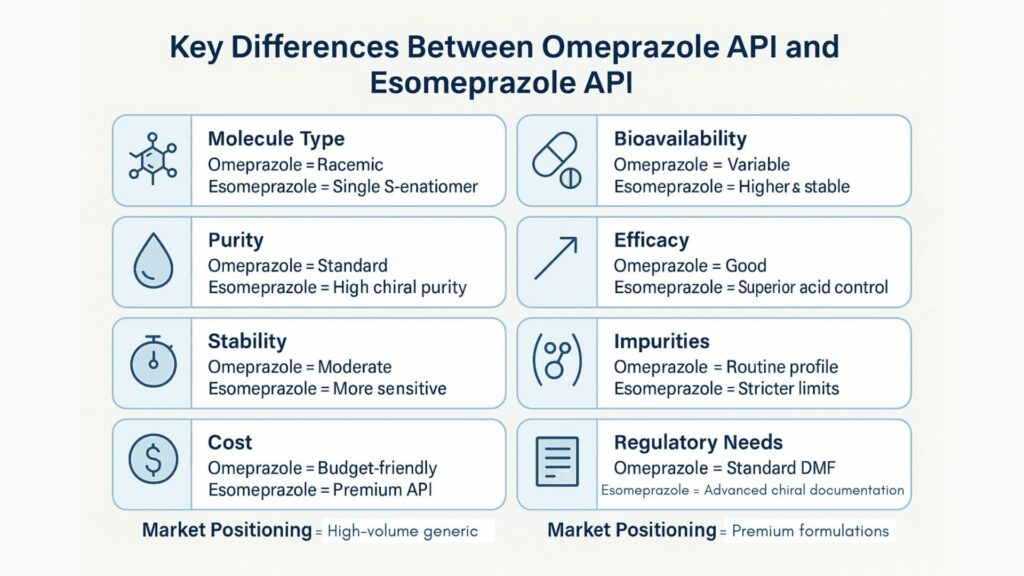
Key Differences Between Omeprazole API and Esomeprazole API
| Parameter | Omeprazole API | Esomeprazole API |
|---|---|---|
| Chemical Nature | Racemic mixture (R + S enantiomers) | Pure S-enantiomer |
| Bioavailability | Moderate, variable by patient | Higher, more consistent |
| Purity Requirements | Standard purity; lower chiral specificity | Very high chiral purity (≥99.8% S-isomer) |
| Clinical Performance | Effective for general GERD | Superior acid suppression; premium therapy |
| Stability | Moderate sensitivity | More sensitive; often used as stable salt forms |
| Impurity Profile | Well-established impurity profile | Stricter control on chiral & organic impurities |
| Formulation Need | Suitable for cost-effective generics | Ideal for premium, regulated-market products |
| Documentation Demand | Standard documentation (DMF/CEP) | Enhanced chiral validation & advanced studies |
| Market Cost | Lower cost; widely available | Higher cost due to complex synthesis |
| Target Market | Mass-market generics | Premium and regulated markets |
Understanding the functional, regulatory, and quality distinctions between Omeprazole and Esomeprazole APIs is essential for selecting the right molecule for your formulations. The points below highlight how each API differs in structure, performance, and market fit.
Which API Should Importers Choose?
Choose Omeprazole API if you want:
A cost-effective PPI for mass-market generics
Stable supply from multiple manufacturers
Consistent quality and well-established pharmacopoeial standards
Lower manufacturing cost for final formulations
Choose Esomeprazole API if you want:
- A premium PPI with superior bioavailability
- Better clinical efficacy and patient outcomes
- Strong positioning in regulated markets
- Higher therapeutic value for chronic GERD patients
Both APIs play a crucial role in the PPI segment. Importers should evaluate quality, supply reliability, regulatory readiness, and final market goals before choosing.
Final Thoughts
Omeprazole API and Esomeprazole API may belong to the same therapeutic class, but their differences, from molecular structure to regulatory complexity, determine their suitability for different formulations and markets.
For importers, understanding these nuances ensures a strategic sourcing decision, helps maintain product quality, and supports compliance in both regulated and semi-regulated markets.
If you need support in sourcing high-quality Omeprazole API or Esomeprazole API, or require detailed technical documentation, Thykn (India) International can assist with reliable information and supplier coordination.




